A Nanobody Specific to Prefusion Glycoprotein B Neutralizes HSV-1 and HSV-2
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are major contributors to global public health challenges. These viruses, along with others like human cytomegalovirus and Epstein–Barr virus, have complex glycoprotein envelopes that play crucial roles in the virus's ability to enter host cells. Among these glycoproteins, glycoprotein B (gB) is the most conserved and vital for membrane fusion during infection.
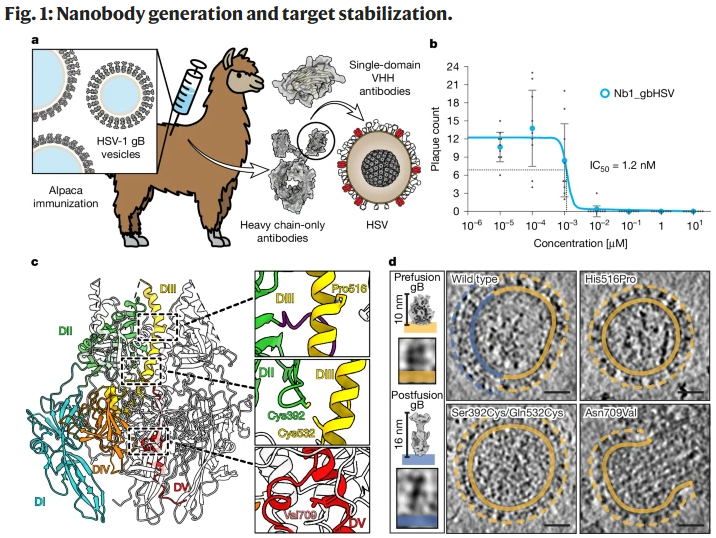
Despite its importance, there have been no antiviral drugs or neutralizing antibodies targeting gB, particularly its prefusion form, due to its metastable and difficult-to-study nature. In this study, researchers isolated prefusion-specific nanobodies that neutralize both HSV-1 and HSV-2. The key breakthrough came from mutational stabilization, which allowed the determination of the full-length prefusion structure of HSV-1 gB. This achievement unlocked previously unresolved structural features of the virus, revealing a new fusion loop arrangement crucial for understanding the fusion process.
The nanobody binds to an epitope spanning three domains that are uniquely proximate only in the prefusion state, preventing the conformational changes necessary for virus entry. By stabilizing gB in its prefusion state, the nanobody effectively neutralizes the virus, offering a potential avenue for antiviral intervention. This discovery paves the way for developing targeted therapeutic strategies against HSV and related viruses by manipulating the virus's fusion machinery at the prefusion stage.
To explore more about how nanobodies can revolutionize antiviral treatments or to discuss potential collaboration opportunities, feel free to reach out to us for more information.
Source: Nature