Nanobody-STING Conjugates: A Game-Changer for Systemic Cancer Immunotherapy
Cancer immunotherapy has changed how we approach tumor treatment—but it still faces serious hurdles. For many patients, existing checkpoint inhibitors (like anti-PD-1 or anti-CTLA-4 therapies) aren't enough, largely due to poor tumor immunogenicity and a hostile, immunosuppressive tumor microenvironment (TME).
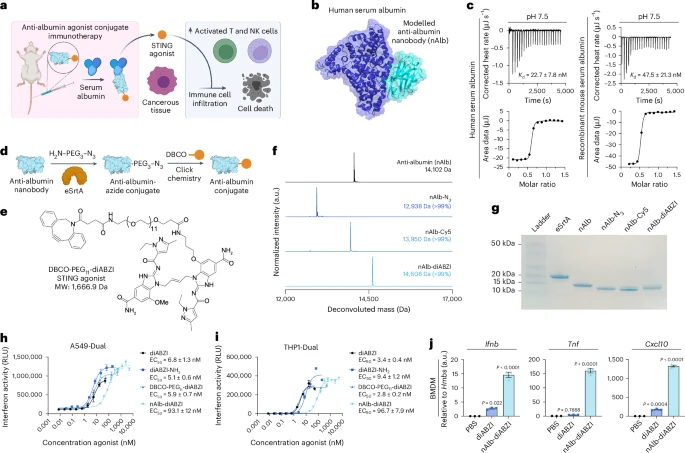
Now, scientists have unveiled an innovative solution that fuses precision targeting with immune activation: albumin-hitchhiking nanobody–STING agonist conjugates.
Why STING? Why Now?
The STING (Stimulator of Interferon Genes) pathway plays a central role in innate immunity by activating type I interferons and pro-inflammatory cytokines—key ingredients for turning a "cold" tumor into a "hot" one.
While STING agonists like cyclic dinucleotides (CDNs) showed great promise in preclinical models, they've underperformed in clinical trials. One key reason? Their poor pharmacokinetics and low accumulation in tumors when delivered systemically.
Enter Nanobodies and Albumin Hitchhiking
Researchers tackled this challenge by chemically linking a STING agonist (diABZI) to a nanobody that binds serum albumin—a naturally long-circulating protein that tends to accumulate at tumor sites.
This design offers multiple advantages:
Longer circulation time
Better tumor localization
Reduced systemic toxicity
Improved pharmacological profile
In mouse models, this approach triggered robust immune activation, including natural killer (NK) cell and T-cell infiltration, leading to significant tumor suppression.
Modular Design with Dual Targeting
Even more exciting, the system is modular. By adding a second nanobody against PD-L1, scientists created a bispecific conjugate that:
Further boosts tumor accumulation
Blocks PD-1/PD-L1 immune checkpoint interactions
Enhances adaptive T-cell responses
Generates long-lasting immunological memory
This combinatorial precision makes the platform highly adaptable for multiple therapeutic strategies, including adoptive T-cell therapy.
Why Nanobodies (VHHs) Matter
This study highlights what we at AlpalifeBio strongly believe: nanobodies (VHHs) are the future of drug delivery. Their small size, stability, and customizable formats make them ideal for next-gen biologics—especially in complex systems like immune modulation and targeted oncology.
Conclusion
The albumin-hitchhiking nanobody–STING agonist platform represents a powerful step forward in systemic cancer immunotherapy. By combining innate immune activation with smart delivery and checkpoint blockade, this strategy unlocks potent antitumor responses in settings where traditional treatments fall short.
As nanobody science continues to advance, we're excited to see more translational innovations bridging precise delivery with durable immune control—exactly what the future of immunotherapy demands.
Source: Nature